SkinnyZymes™ is a broad spectrum proteolytic enzyme formulation, containing pancreatin, bromelain, papain, lipase, amylase, trypsin and alpha chymotrypsin. It may be utilized to support numerous protein metabolism pathways. Proteolytic enzymes are capable of exerting influence over a wide variety of physiological and biochemical processes. The benefits of SkinnyZymes include its effect on muscle soreness and discomfort due to overexertion, the support of hormone processing, as well as providing support for healthy digestive, immune and circulatory functions.
Skinnyzymes Subscription
$29.33 / month
In stock
In stock
Description
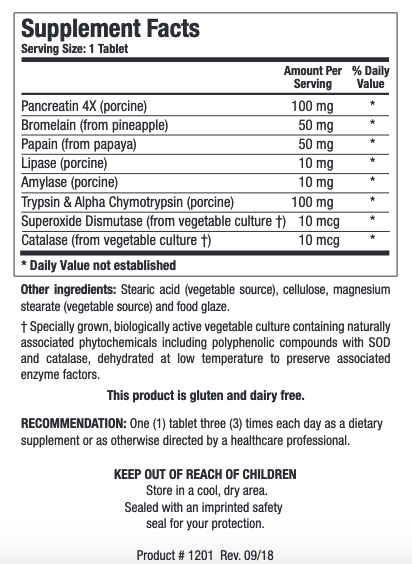
INGREDIENTS:
-
 Pancreatin 4X (porcine)
Pancreatin 4X (porcine)
 Bromelain (from pineapple)
Bromelain (from pineapple) Papain (from papaya)
Papain (from papaya) Lipase (porcine)
Lipase (porcine) Amylase (porcine)
Amylase (porcine) Trypsin & Alpha Chymotrypsin (porcine)
Trypsin & Alpha Chymotrypsin (porcine) Superoxide Dismutase (from vegetable culture†)
Superoxide Dismutase (from vegetable culture†) Catalase (from vegetable culture†) †Specially grown,
Catalase (from vegetable culture†) †Specially grown, biologically active vegetable culture containing naturally associated phytochemicals including polyphenolic compounds with SOD and catalase
biologically active vegetable culture containing naturally associated phytochemicals including polyphenolic compounds with SOD and catalase dehydrated at low temperature to preserve associated enzyme factors. Stearic acid (vegetable source)
dehydrated at low temperature to preserve associated enzyme factors. Stearic acid (vegetable source) cellulose
cellulose magnesium stearate (vegetable source) and food glaze.
magnesium stearate (vegetable source) and food glaze.
This product is gluten and dairy free.
Proteolytic Enzyme Formula
An Outstanding and Highly Effective Treatment for Muscle Soreness and Discomfort Due to the Rigors of Overexertion
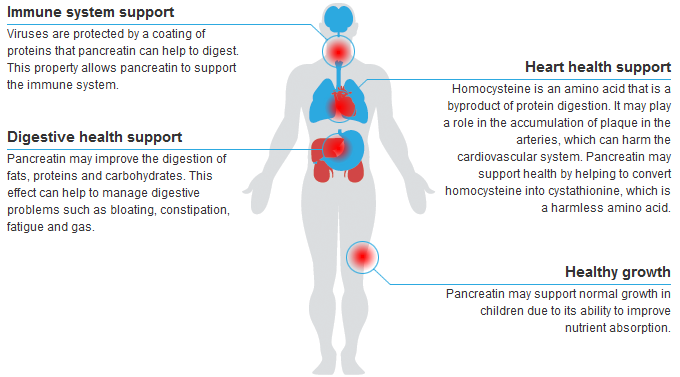
MAJOR BENEFITS
 Supports Hormone Processing
Supports Hormone Processing Digestive Support
Digestive Support Immune System Support
Immune System Support Supports Healthy Circulatory System
Supports Healthy Circulatory System
SkinnyZymes™, a broad spectrum nutritional supplement, is capable of exerting influence over a variety of physiological and biochemical processes. SkinnyZymes™ is unique because of its proteolytic enzyme formulation that supports the numerous pathways of protein metabolism.
For nearly 40 years, proteolytic enzymes have provided safe, effective, reliable results.
Simply put, SkinnyZymes™ works!
FUNCTIONS OF PROTEOLYTIC ENZYMES
Proteolytic enzymes occur widely in plants, animals and microorganisms, where they catalyze the hydrolysis of peptide bonds of proteins. The ultimate products of proteolytic degradation are amino acids. Many proteases in the body produce protein fragments (peptides), that possess physiologic activity. Actions of proteolytic enzymes include: digestion, blood clotting, hormone and cytokine processing (such as insulin), growth hormone, interleukin 1-B, endorphins, enkephalins, renin, kinins, remodeling of connective tissue, and lysosomal degradation. Even apoptosis (programmed cell death) employs proteolytic enzymes (ICE-like proteases) which degrade constituents of the cellular cytoskeleton.
PROTEOLYTIC ENZYMES IN DIGESTION
A wide assortment of proteolytic enzymes are required to degrade food proteins to amino acids. Digestive enzymes are manufactured as inactive precursors called zymogens, which must first be activated. Ingested proteins first encounter proteolytic enzymes in the stomach, which produces pepsin. Pepsin refers to a closely related group of proteases produced by the gastric mucosa from its precursor, (pepsinogen), by HCl. Pepsins are classified as
SkinnyZymes.
endopeptidases because they leave peptide bonds within protein chains. Upon leaving the stomach, the acidic chyme is neutralized in the intestine by sodium biocarbonate from pancreatic secretions, and subjected to a battery of powerful proteases. The acid in chyme triggers pancreatic secretions; therefore, hypochlorhydria may be related to pancreatic insufficiency.
The exocrine pancreas produces a battery of potent endopeptidases, such as trypsin and alpha chymotrypsin, as their zymogens (trypsinogen and chymotrypsinogen, respectively). Trypsin possesses a very high degree of peptide bond specificity; it cleaves bonds adjacent to arginine and lysine only. Chymotrypsin cleaves peptide bonds adjacent to large, non-polar amino acids, such as aromatic amino acids and methionine. Other pancreatic proteases include elastase. In contrast, pancreatic expopeptidases, represented by carboxypeptidases, cleave amino acids from the carboxyl terminus of peptides. Carboxypeptidases are derived from the zymogens,procarboxypeptidase A and B. Aminopeptidases are produced by the intestinal mucosa. These digestive enzymes hydrolyze off amino acids sequentially from the N terminus of peptides. Trypsin activates most of the zymogens; trypsin is converted from trypsinogen by the entericenzyme enteropeptidase. Thus, the activation cascade of zymogens occurs after secretion into the intestine to prevent premature activation of pancreatic proteases.
INGREDIENTS OF SkinnyZymes
Pancreatin is a preparation of porcine pancreas, highly enriched in pancreatic enzymes, including trypsin, chymotrypsin, carboxypeptidase, amylase (starch digestion), and lipase (fat digestion). Porcine pancreatin contains these enzymes in a ratio similar to human pancreas.The activity of digestive enzymes from human and porcine pancreas are similar. Pancreatic enzymes can be denatured by exposure to gastric acid; therefore, SkinnyZymes is coated to preserve these proteolytic activities during transit through the gastrointestinal tract
The benefits of pancreatin in supporting digestive function has been reported.
- Excessive amounts of pancreatic enzymes can cause thickening of the colon in children with cystic fibrosis.
- Bromelain is a sulfhydryl protease obtained from the stems and fruit of the pineapple. This enzyme is an endopeptidase with broad amino acid specificity, permitting it to hydrolyze many soluble proteins as found in food. It is active over the range of 4 to 9. Bromelain is activated by reducing agents such as cysteine and inhibited by heavy metals. It is a glycoprotein, and the carbohydrate moiety may confer enzyme stability. The arachidonic acid cascade leads to the production of proinflammatory eicosanoids. In an animal model system, bromelain interfered with the arachidonic acid cascade.
- In vitro experiments showed that bromelain and papain blocked the formation of immune complexes in vitro
- and a similar effect was noted in rabbits with orally administered pancreatin, bromelain and papain.
- Bromelain has been reported to support the restoration of normal function in skeletal muscle.
- Papain is obtained from the latex of the green fruit and leaves of papaya. It is active from pH 3 to 10.5, with an optimum between pH 5 and 7. Therefore, both papain and bromelain are relatively stable to pH ranges occurring in the GI tract. Papain is also a sulfhydryl enzyme and is activated by reducing agents, such as reduced cysteine. On the other hand, metal ions such as iron, zinc, copper, and heavy metals, such as lead and mercury, inhibit papain. Like bromelain, papain possesses a broad specificity for peptide cleavage; it will hydrolyze peptide bonds of many different amino acids.
Intestinal Uptake of Proteins and Peptides
The sum of the activities of multiple digestive enzymes assures that the overall effect will be to break down many denatured food proteins. However, not all ingested proteins are completely degraded. Highly fibrous proteins such as collagen, elastin and keratins resist proteolytic attack. In addition, undenatured globular proteins resisted gradation. For example, chymotrypsin itself resists intestinal proteolytic degradation, and appreciable levels of chymotrypsin are excreted. - In addition, orally administered proteins and antigens can be absorbed by the intestine.
- Absorption of maternal antibodies by an infant’s intestines is a well-established example. Bromelain labeled with 125-iodine was used to follow uptake in rats.
- Absorption was confirmed. After 1 hour, radioactivity was recovered in a protein corresponding to native bromelain. Furthermore, the absorbed bromelain was preferentially localized at sites of inflammation.
The gut-associated immune system receives antigenic macromolecules from the intestinal lumen. Specialized enterocytes called M cells transport macromolecules through the epithelium to the lamina propria. IgA, synthesized by plasma cells of the lamina propria in response to these antigens, is then exported by the gut epithelium as secretory IgA to confer specific protection to the gut mucosa. Other transport processes exist. Thus macromolecules, including intact proteins, can be transported across the epithelial cell membranes by transcellular mechanisms, followed by phagocytosis of the foreign material. Some of the vesicular material fuses with lysosomes to be degraded within phagolysosomes by lysosomal proteases. The remainder passes from enterocytes through the basolateral membrane into the interstitial space, from which it may become available for macrophages and lymphoid cells, or it may pass into the blood or lymph. - Orally administered enzymes consumed on an empty stomach may have a greater likelihood of being absorbed. Coated bromelain, trypsin and chymotrypsin are reported to support normal maintenance and repair. On the other hand, proteolytic enzymes consumed with a meal would be expected to act as digestive support for normal proteolytic degradation of food proteins.
PROTEOLYTIC ENZYMES IN INFLAMMATION
Mast cells contain large amounts of histamine and heparin as well as proteolytic enzymes that participate in inflammation. Release of these agents from mast cells is triggered by chemical agents, antibody-antigen reactions and certain drugs. A variety of substances besides histamine act as vasodilators, which increase capillary permeability to leukocytes and cause pain. In particular, kinins such as bradykinin and kallidin represent vasodilator polypeptides. Bradykinin is a peptide produced by the enzyme kallikrein from its alpha 2-globulin substrate. Tissue kallikreins may be activated and released by trauma, inflammation, toxins and heat. Trypsin-like proteases behave like kallikrein to release kinins. Other endogenous proteolytic enzymes destroy kinins: Bradykinin is destroyed by kininase I and II (angiotensin I converting enzyme). The balance between kinin formation and inactivation is also controlled by kallikrein inhibitors in the blood.
Proteolytic enzymes are involved in vasoconstriction as well as vasodilation. Both angiotensin, a potent vasoconstrictor, and bradykinin are released from different precursor proteins by proteolytic enzymes. On the other hand, the converting enzyme (proteolytic enzyme) of the angiotensin system is a powerful inactivator of bradykinin. Possibly exogenous proteolytic enzyme may destroy bradykinins. Proteolytic enzymes of kinin activation also have a role in blood clotting, thus plasma kallikrein activates factor XII (Hageman factor), a protease required in the blood clotting scheme.
Activity of Proteolytic Enzymes
Pancreatin. Measurement of proteolytic activity of pancreatin has been defined by the U.S. Pharmacopoeia based upon the digestion of a standard protein, casein. Pancreatin 4X possesses 4 times the activity of pancreatin 1X (100 USP units of proteolytic activity per milligram). Therefore, 100 mg of pancreatin 4X per tablet of SkinnyZymes provides 10,000 USP units.
SkinnyZymes™ contains 100 mg of trypsin/chymotrypsin per tablet.
These statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure, or prevent any disease.
© Copyright 2016
LIT-093 Rev. 07/16
Trypsin. The USP assay for trypsin is based upon the hydrolysis of a synthetic substrate for trypsin. The amount of trypsin in SkinnyZymes™ is equivalent to 8,000 USP units. In addition, pancreatin itself contains a substantial amount of trypsin activity (10,000 USP units). The total trypsin activity in a single tablet of SkinnyZymes™ is 18,000 units.
Chymotrypsin. Chymotrypsin is also a component of pancreatin; typically, there are 5 units of trypsin activity per unit of chymotrypsin in pancreatin. Papain. Papain activity is based upon the hydrolysis of casein. The amount of papain in SkinnyZymes is equivalent to 50 mg USP papain per tablet, representing 300,000 USP papain units.
Bromelain. SkinnySymes™ provides 50 mg of bromelain, standardized to 1,000 MCU (milk clotting units) per gram, similar to 50 mg of papain USP. (The U.S. Pharmacopoeia has not yet established a standard assay for bromelain.)
REFERENCES
1. Guarner L; Rodriguez; Guarner F and Malagelada JR. Fate of oral enzymes in pancreatic insufficiency. Gut 1993; 34: 708-712.
2. Mac Sweeney EJ; Oades PJ; Buchdahl R; Rosenthal M and Bush A. Relation of thickening of colon wall to pancreatic-enzyme treatment in cystic fibrosis. Lancet 1995; 345: 752-756.
3. FitzSimmons SC et al. High-dose pancreatic-enzyme supplement and fibrosingcolonopathy in children with cystic fibrosis. N Engl J Med 1997p 336: 1283-1289.
4. Vellini M et al. Possible Involvement of Eicosanoids in the Pharmacological Action of Bromelain. Arzneimittel Forschung 1986; 36 (1): 110-112.
5. Gebauer F; Stauder G and Kunze R. Proteolytic Enzymes Modulate Preformed, Fixed Immunocomplexes and the Process of Immunocomplex-Formation InVitro. Nutr Immun 1995; 3 (3): 19.
6. Steffen C and Menzel J. In-vivo-Abbau von Immunkomplexen in der Niere durchoral applizierte Enzyme. Weinerklnishe Wochenschrift 1987; 99 (15): 525 531.
7. Walker JA; Cerny FJ; Cotter JR and Burton HW. Attenuation of contraction induced skeletal muscle injury by bromelain. Med Sci Sports Exerc 1992; 24 (1):20-25.
8. Goldberg DM. Enzymes as agents for the treatment of disease. Clinica ChimicaActa 1992; 206: 45-76.
9. Coleny PC and Neutra MR. Macro molecular transport in the fetal rat intestine. Gastroenterology 1985; 89: 294-306.
10. White RR; Crawley FEH; Vellini M and Rovati LA. Bioavailability of 125I Bromelain after Oral Administration to Rats. Biopharma & Drug Disposition 1988; 9: 397-403.
11. Mestecky J and McGhee JR. Oral Immunization: Past and Present. Current Topics in Microbiology and Immunology 1989; 146: 3-1.